Introduction
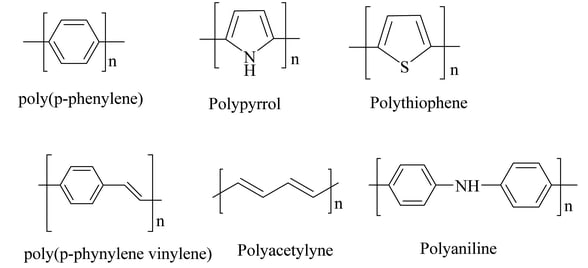
Conducting polymers are a class of organic polymers capable of conducting electricity. They combine the processing advantages of plastics with the electrical properties of metals or semiconductors. Typically synthesized via chemical or electrochemical polymerization, their electrical conductivity can be significantly enhanced through a process known as doping. Prominent examples include polyacetylene, polypyrrole, and polyaniline.
-
Polyacetylene: One of the earliest discovered conducting polymers, notable for its high conductivity upon doping.
-
Polypyrrole (PPy): Widely researched for its stability and range of applications, especially in sensors and actuators.
-
Polyaniline (PANI): Known for good environmental stability, ease of synthesis, and tunable conductivity.
Definition
Conducting polymers are organic polymers that exhibit electrical conductivity due to the delocalization of π-electrons along their conjugated backbone.
- They exhibit either metallic conductivity or semiconducting behavior.
- Offer a unique balance between ease of processing and desirable electrical performance.
Synthesis and Doping
Conducting polymers are generally synthesized through chemical or electrochemical polymerization. Chemical methods are more commonly used due to their cost-effectiveness and scalability.
Doping is the process of introducing dopant molecules to modify the electrical conductivity of the polymer. This is a critical step in transforming an insulating polymer into a conductive one.
Conditions for Conductivity in Polymers
-
Conjugated π-Electron System: The polymer backbone should have alternating single and double bonds (conjugation), allowing delocalized π-electrons to move freely along the chain.
-
Doping: Conductivity typically requires doping (either oxidation or reduction) to introduce charge carriers (electrons or holes) that can move through the conjugated system.
-
Planar Structure: A planar backbone helps maintain effective overlap of p-orbitals, which is essential for delocalized electron movement.
-
Interchain Interactions: Efficient electron transport also depends on how well polymer chains are packed; better alignment allows for easier charge transfer between chains.
Applications
-
Electronics: Used in devices like organic light-emitting diodes (OLEDs), solar cells, and flexible electronics due to their conductivity and optical properties.
-
Biomedical: Their biocompatibility and customizable properties make them ideal for biosensors, neural interfaces, and drug delivery systems.
-
Energy Storage: Utilized in supercapacitors and batteries due to their pseudocapacitive behavior, allowing for efficient charge storage and transfer.
-
Other Applications: Smart textiles, actuators, antistatic coatings, and hydrogels are among other domains benefiting from conducting polymers.
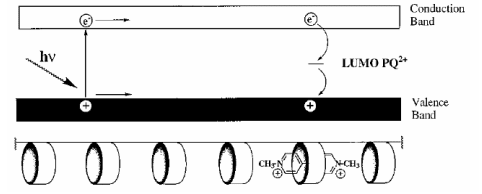
Mechanism of conduction in Polyacetylene
The conduction mechanism in polyacetylene is based on the delocalization of π-electrons along its conjugated polymer chain, which becomes electrically active through the process of doping. Doping introduces charge carriers (electrons or holes) that enable current flow by moving along the backbone of the polymer.
1. Conjugated Structure:
Polyacetylene has a linear backbone with alternating single and double carbon-carbon bonds, forming a conjugated system. This structure enables π-electrons to become delocalized across the entire chain, allowing for enhanced electronic interaction and mobility.
2. Doping Process:
In its pure form, polyacetylene is an electrical insulator. To make it conductive, it must be doped with either:
- Electron acceptors (p-type doping)
- Electron donors (n-type doping)
3. P-type Doping (Oxidative Doping):
When polyacetylene is exposed to an electron acceptor such as iodine (I₂), electrons are removed from the polymer chain. This results in the formation of positively charged species, known as holes or polarons, which act as mobile charge carriers.
4. N-type Doping (Reductive Doping):
Conversely, when an electron donor is introduced, electrons are added to the polymer chain. This produces negatively charged polarons, which also contribute to charge transport.
5. Charge Carrier Mobility:
Once doping occurs, these charge carriers (holes or electrons) can move freely along the conjugated backbone of the polymer. This movement under an applied electric field enables electrical conductivity.
In essence, doping transforms polyacetylene from an insulator to a conductor by creating mobile charge carriers within its delocalized π-electron system.
Polymers
Introduction, Classification, Glass transition temperature, factors affecting Tg and its significances, Synthesis, properties and applications of PP, PVC, PMMA, polyurethanes, Epoxy resins, Silicon Rubber, PET, Lexan, Kevlar.
Nano Materials
Introduction, Properties of nanomaterials, Graphene, Fullerenes, Carbon nanotubes, nano wires, nano cones, Application of nano-materials.