Introduction
Water is nature's most wonderful, abundant, and useful compound. While humans can survive for a number of days without food, water is so essential that survival without it is impossible. Not only is water essential for the lives of animals and plants, but it also holds a unique position in industries. Perhaps its most important application as an engineering material is in steam generation. Water is also used as a coolant in power and chemical plants.
Water is widely distributed in nature. It is estimated that about 75% of the Earth's surface consists of water. In addition to visible water on Earth, there is a large amount of water beneath the Earth's surface, with an average depth of over three kilometers. The air contains 12 to 15% of water vapor by volume. Water is also found in living things; the human body consists of about 60% water, while plants, fruits, and vegetables contain 90 to 95% water.
Characterstics of Water
According to recommendations by the World Health Organization (WHO) and the Indian Council of Medical Research (ICMR), the following are the important characteristics of potable water:
- It should be clear, colorless, and odorless.
- It should be cool and pleasant to taste.
- It should be free from harmful bacteria and suspended impurities.
- It should be free from dissolved gases such as , , and , as well as poisonous minerals like lead, arsenic, and manganese.
- Hardness should be less than 500 ppm.
- Chloride ion content should be less than 250 ppm.
- The pH of the potable water should be between 6.5 and 8.5.
Sources
- Surface Water
- Underground Water
Surface Water
-
Rainwater: Rainwater is the purest form of natural water, obtained through evaporation from the Earth's surface. However, during its descent through the atmosphere, it absorbs significant amounts of gases such as , , , etc., as well as suspended solid particles.
-
River Water: Generally, the more contact that water has with soil or soluble minerals in the soil, the greater the amount of dissolved impurities in river water. Therefore, river water contains dissolved minerals from the soil, such as chlorides, sulphates, bicarbonates of calcium, magnesium, and iron. Additionally, river water contains organic matter from decomposed plants, as well as small particles of sand and rock in suspension. Consequently, river water contains considerable amounts of both dissolved and suspended impurities.
-
Lake Water: Lake water typically maintains a more constant composition. It usually contains fewer dissolved minerals than even well water, but its organic matter content is relatively high.
-
Sea Water: Sea water is the most impure form of natural water, containing an average of about 3.5% dissolved salts, primarily sodium chloride (about 2.6%). Other salts present include sodium sulphate, potassium bicarbonate, magnesium bicarbonate, and calcium bicarbonate, among others.
Surface water generally contains suspended matter, often containing disease-producing bacteria. Therefore, such water is not considered safe for human consumption. Many surface water sources, such as lakes and rivers, are used for drinking water after appropriate treatment to remove contaminants.
Underground Water
A portion of rainwater that reaches the Earth's surface percolates into the ground. As this water travels downward, it interacts with various mineral salts present in the soil, dissolving some of them. The water continues its descent until it encounters a hard rock, at which point it may reverse direction and ascend, emerging in the form of a spring or well. Generally, underground water appears clear due to the soil's filtering action but contains higher levels of dissolved salts, resulting in greater hardness. Underground water typically has high organic purity.
Common Impurities In Water
-
Dissolved Solids: These can include minerals such as calcium, magnesium, sodium, and potassium, as well as salts like chloride, sulphate, and bicarbonate.
-
Organic Matter: This can be derived from decaying plant or animal material, as well as from human or animal waste. Organic matter can contribute to unpleasant tastes and odors in water, as well as provide a medium for bacterial growth.
-
Microorganisms: Bacteria, viruses, and parasites can contaminate water sources, potentially causing waterborne diseases such as cholera, typhoid fever, and giardiasis.
-
Chemical Contaminants: These can include pollutants such as pesticides, herbicides, industrial chemicals, and heavy metals like lead, arsenic, mercury, and cadmium. These contaminants may originate from agricultural runoff, industrial discharges, or improper disposal of household chemicals.
-
Suspended Solids: These are particles that are not dissolved in water but remain suspended, such as silt, clay, and organic matter. Suspended solids can cause cloudiness in water and may harbor bacteria or other pathogens.
Hardness of Water
Topic asked in September 2021 (CBCS) question paper.
Hardness is caused by the soluble salts of calcium, magnesium, iron, manganese, sodium, sulphates, chlorides, and nitrates. The degree of hardness depends on the type and amount of impurities present in the water. Hardness also depends on the amount of carbon dioxide in solution. Carbon dioxide influences the solubility of the impurities that cause hardness. The hardness caused by carbonates and bicarbonates is called carbonate hardness. The hardness caused by all other substances (chlorides, sulphates, nitrates) is called non-carbonate hardness,
Hard water
Topic asked in May 2017 (CBS) question paper.
Water that does not produce lather with soap solution but instead produces a white precipitate (scum) is called hard water. In other words, hard water contains mineral salts, such as calcium and magnesium ions, which limit the formation of lather with soap.
The reaction between soap (sodium stearate) and calcium ions reduces the effectiveness of soap in hard water, leading to reduced lathering and the formation of scum.
Hard water contains high concentrations of minerals, particularly calcium and magnesium ions. When soap is added to hard water, these ions react with the soap to form insoluble salts called calcium and magnesium soaps. These soaps are not effective for cleaning and can form a scum or residue on surfaces, including your skin and clothes.
To overcome this, more soap is needed to compensate for the reaction with the minerals in the water. This is why hard water requires more soap to produce a satisfactory lather and cleaning effect compared to soft water, which contains fewer minerals. Additionally, the minerals in hard water can also reduce the effectiveness of other cleaning agents, like detergents, requiring higher concentrations to achieve the desired cleaning results.
Formation of Hard Water
Hard water forms due to the presence of minerals like calcium and magnesium that are not removed or separated by sedimentation or filtration. When hard water reacts with soap (sodium salts of stearic acid or palmitic acid), it produces a curdy precipitate.
In the above reactions, hard water reacts with the sodium salt of stearic acid to form insoluble calcium stearate or magnesium stearate, which separates out without producing lather.
How to Detect Hardness?
Hardness of water can be detected in two ways:
-
Soap Test: When water is treated with a soap solution, if it prevents lathering and forms a white scum, the water contains hardness.
-
Eriochrome Black-T Indicator Test: Water containing hardness gives a wine-red color when tested with Eriochrome Black-T indicator. The total water hardness (including both Ca2+ and Mg2+ ions) is measured in parts per million (ppm) or weight/volume (mg/L) of Calcium Carbonate in the water. Although water hardness usually measures only the total concentrations of calcium and magnesium (the two most prevalent divalent metal ions), iron, aluminum, and manganese may also be present at elevated levels in some geographical locations. The predominant source of magnesium is dolomite .
Types of Hardness
Topic asked in July 2022 (CBCS) , September 2021 (CBCS) question paper.
Temporary Hardness
Temporary hardness of water is caused by calcium and magnesium bicarbonates. This can be removed by simply boiling the water. Boiling converts bicarbonates into carbonates, which precipitate as insoluble solids.
Temporary hardness can also be removed by adding hydrated lime to precipitate insoluble carbonates.
Permanent Hardness
Permanent hardness is caused by the presence of soluble salts of calcium and magnesium other than bicarbonates, such as chlorides and sulphates. Permanent hardness cannot be removed by boiling water or using hydrated lime. It can be eliminated by water softening techniques like the Lime-soda process, Zeolite , Ion-exchange resin , reverse osmosis, etc.
Degree of Hardness
Topic asked in September 2021 (CBCS) question paper.
Hardness is typically expressed in a unit known as the degree of hardness, which is commonly measured in terms of calcium carbonate equivalents due to the ease of precipitation of . The degree of hardness can be calculated using the following formulas:

or
Equivalent of =
Units of Hardness:
-
Parts per million (ppm): This represents the number of equivalent parts of present per million parts of water by weight.
-
Milligrams per liter (mg/l): This indicates the number of milligrams of present in one liter of water.
-
Degree Clarke (°Cl): This measures the number of equivalent parts of present per 70,000 parts of water.
-
Degree French (°Fr): This denotes the number of equivalent parts of present per 105 parts of water.
Correlation between ppm, mg/l, °Cl, and °Fr:
1 ppm = 1mg/l = 0.07 °Cl = 0.1 °Fr
Estimation of Hardness by EDTA Method
Topic asked in September 2021 (CBCS) question paper.
EDTA forms a colorless stable complex with and ions in water at pH 10. Ammonia buffer is used to maintain the pH. In this method, EBT (Eriochrome Black-T) is used as an indicator. Initially, EBT forms an unstable complex with and ions, giving a wine-red color to the solution. During the titration, EDTA reacts with this complex (Ca-EBT or Mg-EBT complex), forming a stable complex (Ca-EDTA or Mg-EDTA) and releasing the blue EBT into the solution. Hence, the endpoint is indicated by a change from wine red to blue color.
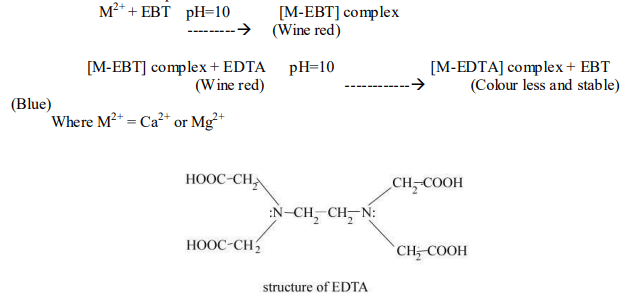
Various steps involved in this method are:
-
Preparation of Standard Hard Water: Prepare a solution with 1 mg of per 1 mL of water.
-
Standardization of EDTA Solutions: Pipette out 50 mL of standard hard water into a conical flask. Add 10-15 mL of buffer solution and 4 to 5 drops of EBT indicator. Titrate with EDTA solution until the wine-red color changes to clear blue. Note the volume used as mL.
-
Titration of Unknown Hard Water: Titrate 50 mL of the water sample as indicated above. Note the volume used as mL.
-
Titration for Permanent Hardness: Take 250 mL of the water sample in a large beaker. Boil it until the volume is reduced to about 50 mL. Filter and wash the precipitate with distilled water, then collect the filtrate and washings in a 250 mL measuring flask. Finally, make up the volume to 250 mL with distilled water. Titrate 50 mL of the boiled water sample as in step (2), and note the volume of EDTA used as mL.
Calculations:
50 mL of standard hard water corresponds to mL of EDTA, and since 50 mg of is equivalent to mL of EDTA, we have: 1 mL of EDTA = mg of .
Now, for the given hard water sample, the volume of EDTA used is mL, which corresponds to:
Total hardness of water = mg/L = ppm.
Similarly, for the boiled water sample, the volume of EDTA used is mL, which corresponds to:
Permanent hardness = mg/L = ppm.
And finally, the temporary hardness can be calculated as the difference between total hardness and permanent hardness, given by:
Temporary hardness = Total hardness - Permanent hardness = ppm.
Water Quality Parameters
Water quality parameters are characteristics or properties of water that are measured to assess its suitability for various uses, including drinking, agriculture, industry, and aquatic ecosystems. These parameters provide valuable information about the physical, chemical, and biological characteristics of water and help determine its safety, purity, and overall health.
Categories of Water Quality Parameters:
- Physical Parameters (Turbidity)
- Chemical Parameters (Total Dissolved Solids (TDS), Hardness, Chlorine and Arsenic Test)
- Biological Parameters (BOD, COD)
Turbidity
Turbidity measures the cloudiness or haziness of water due to suspended particles. It is measured using a turbidimeter or nephelometer, which measures the amount of light scattered by suspended particles in the water. The unit of measurement is usually nephelometric turbidity units (NTU). High turbidity can affect aquatic ecosystems, interfere with water treatment processes, and indicate the presence of contaminants. Turbidity in water can be removed through various physical, chemical, and biological methods, depending on the source and severity of turbidity. Some common techniques for turbidity removal are: Settling and Filtration, Coagulation and Flocculation.
Total Dissolved Solids (TDS)
Topic asked in July 2022 (CBCS) and May 2017 (CBS) question paper.
TDS refers to the total concentration of dissolved substances in water, including minerals, salts, metals, and other organic and inorganic compounds. Elevated TDS levels can affect water taste, clarity, and suitability for drinking and irrigation. TDS can be measured through various methods, including gravimetric analysis, conductivity measurement, and optical methods. Gravimetric analysis involves evaporating a measured volume of water and weighing the remaining solids to determine total dissolved solids. Conductivity measurement relies on the fact that dissolved ions in water conduct electricity. Optical methods use spectrophotometry to measure the absorbance of light by dissolved substances. Some common methods used for TDS removal are: Reverse Osmosis (RO), Distillation.
Hardness
Water hardness refers to the concentration of calcium and magnesium ions dissolved in water. These ions are derived from the weathering of rocks and soils.
Types of Hardness:
-
Temporary hardness is caused by the presence of bicarbonate ions () of calcium and magnesium. Boiling the water can remove temporary hardness because the bicarbonate ions decompose and form insoluble carbonate salts that precipitate out.
-
Permanent hardness is caused by the presence of dissolved calcium and magnesium salts other than carbonates, such as sulphates and chlorides. Boiling does not remove permanent hardness. It removed through various water softening techniques.
Chlorine Test
Chlorine is commonly used as a disinfectant in water treatment to kill bacteria and other pathogens. It exists in water in various forms, including free chlorine (hypochlorous acid and hypochlorite ions) and combined chlorine (chloramines).
Analysis Methods:
-
DPD Method: This method uses a chemical compound called N,N-diethyl-p-phenylenediamine (DPD) to react with free and combined chlorine, forming a colored compound. A colorimeter measures the intensity of color produced by the DPD reaction with chlorine.
-
Amperometric Titration: In this method, chlorine is oxidized at an electrode surface, generating an electric current that is proportional to the chlorine concentration. This method is particularly useful for continuous monitoring of chlorine levels in water treatment plants.
Arsenic Test
Arsenic is a toxic metalloid that can contaminate water sources through natural geological processes or industrial activities. Long-term exposure to arsenic in drinking water can lead to serious health issues.
Analysis Methods:
-
Colorimetric Methods: These methods involve reacting arsenic in the water sample with reagents that produce a colored compound, such as in the Gutzeit method. The intensity of color is measured using a colorimeter or spectrophotometer and is proportional to the arsenic concentration.
-
Atomic Absorption Spectroscopy (AAS): AAS directly measures the concentration of arsenic in a water sample by analyzing the absorption of light at specific wavelengths by arsenic atoms. This method provides accurate and precise results but requires specialized equipment and skilled personnel.
BOD (Biochemical Oxygen Demand)
Topic asked in July 2022 (CBCS) and September 2021 (CBCS) question paper.
BOD measures the amount of dissolved oxygen consumed by microorganisms while decomposing organic matter in water. It is an indicator of organic pollution and the water's ability to support aquatic life.
Analysis Method:
- Standard BOD Test: This method involves incubating a water sample in a sealed bottle for a specified period (usually 5 days) at a constant temperature (20°C). The initial and final dissolved oxygen concentrations are measured, and the difference indicates the BOD of the sample.
COD (Chemical Oxygen Demand)
Topic asked in July 2022 (CBCS) and September 2021 (CBCS) question paper.
COD measures the amount of oxygen required to chemically oxidize organic and inorganic substances in water. It provides a more rapid assessment of water pollution compared to BOD.
Analysis Methods:
-
Open Reflux Method: This method involves heating a water sample in the presence of a strong oxidizing agent (such as potassium dichromate) under reflux conditions. The amount of oxygen consumed during the reaction is determined by titration with a reducing agent, such as ferrous ammonium sulphate.
-
Closed Reflux Method: Similar to the open reflux method, but the reaction takes place in a closed system to prevent the loss of volatile substances. This method is suitable for samples containing volatile organic compounds.
Water Softening Techniques
Topic asked in July 2022 (CBCS) , December 2022 (CBCS) , September 2021 (CBCS) , May 2021 (CBCS) and May 2017 (CBS) question paper.
Water softening is the process of removing calcium and magnesium ions, which cause hardness, from water.
Zeolite or Permutit Process
Zeolite is a three-dimensional silicate with the chemical formula hydrated sodium aluminum silicate, represented as (where x = 2-10 & y = 2-6). Zeolites are capable of exchanging ions with sodium ions, making them effective for removing hardness-producing ions from water. This process is commonly known as the permutit process. Zeolite can be represented as , where two Na+ ions are replaced by one or ion.
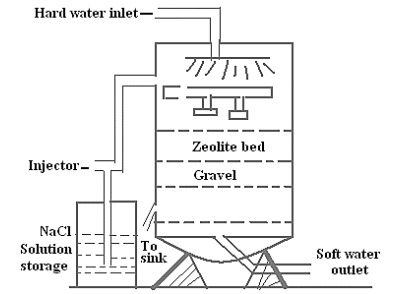
Process
The apparatus consists of a cylindrical metallic vessel with several beds inside containing zeolite salt. Raw water is poured into the apparatus through an inlet, passing through the beds where chemical ion exchange reactions take place. In this process, sodium ions in the zeolite are exchanged with calcium or magnesium ions present in the water, resulting in softened water. After a certain period of use, the zeolite becomes exhausted, meaning all ions are replaced by or , making it ineffective for water softening.
Regeneration
Exhausted zeolite can be regenerated by treating it with a brine solution (10% NaCl solution). The brine solution replaces the calcium or magnesium ions with sodium ions again, restoring the zeolite's softening capacity.
After washing the exhausted zeolite with cold water, and are removed, leaving behind regenerated zeolite ready for reuse.
Limitations of Zeolite Process
-
Turbidity: If the supplied water is turbid, suspended matter must be removed before admitting the water to the zeolite bed. Otherwise, the turbidity will clog the pores of the zeolite bed, rendering it inactive.
-
Presence of Colored Ions: Large quantities of colored ions such as and must be removed first. These ions can form manganese and iron zeolites that are difficult to regenerate.
-
Mineral Acids: If mineral acids are present in water, they can destroy the zeolite bed. Therefore, they must be neutralized with soda before water is admitted to the zeolite softening plant.
Advantages of Zeolite Process
-
Zeolite process removes hardness almost completely, producing water with about 10 ppm hardness.
-
The equipment used is compact, occupying minimal space.
-
No impurities are precipitated, eliminating the risk of sludge formation in the treated water at large scales.
-
The process automatically adjusts for variations in the hardness of incoming water.
-
It requires less time for softening compared to other methods.
Disadvantages of Zeolite Process
-
Treated water contains more sodium salts than in the lime-soda process.
-
The method only replaces and ions with Na+ ions, leaving acidic ions and in the softened water. This can lead to issues such as corrosion and caustic embrittlement in boiler systems.
-
High turbidity water cannot be efficiently treated by this method as fine impurities can deposit on the zeolite bed, causing operational problems.
Ion-exchange resin Process or de-ionization or de-mineralization process
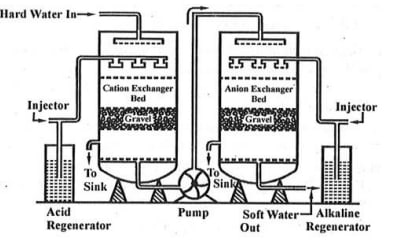
In this process, cations and anions are effectively removed by passing impure water through two separate columns. The first column contains strong acid cation exchange resin with sulfonic acid groups (), which can exchange cations such as calcium (), magnesium (), and sodium ().
Meanwhile, the second column contains strong base anion exchange resin with functional groups like , which acts as an anion exchanger by removing anions such as chloride () and sulfate () from the water.
Chemical Reactions (Anion Exchange):
The removal of ions from the first column and ions from the second column react to form water ().
Regeneration
When both columns are exhausted (capacity for ion exchange is reached), they are treated with separate solutions. The cation exchange resin is regenerated with dilute sulphuric acid or HCl (to generate ions), and the anion exchange resin is regenerated with aqueous NaOH (to generate ions). his process restores the ion exchange capacity of the resins.
For cation exchange resin regeneration:
And for anion exchange resin regeneration:
Advantages of Ion-Exchange Process
- The process can effectively soften highly acidic or alkaline waters, making it suitable for a wider range of water qualities.
- It produces water with very low hardness (around 2 ppm), making it ideal for treating water used in high-pressure boilers where minimal hardness is crucial.
Disadvantages of Ion-Exchange Process
- The equipment for a two-bed ion-exchange system is generally more costly compared to a zeolite softener. Additionally, the regeneration chemicals (sulphuric acid, hydrochloric acid, and sodium hydroxide) can add to the operating expense.
- The efficiency of the process is reduced if the water contains high turbidity (suspended particles). The turbidity level must be below 10 ppm. If it exceeds this limit, turbidity must be removed first through coagulation and filtration processes before the ion-exchange treatment.
Drinking Water Purification
The natural water obtained from rivers, canals, lakes, etc., does not conform to the quality standards prescribed for domestic or municipal water and, as such, is not suitable for municipal supply. It needs to be treated appropriately. Public drinking water systems use different water treatment methods to provide safe drinking water for their communities. Public water systems often employ a series of water treatment steps that include coagulation, flocculation, sedimentation, filtration, and disinfection.
Water Treatment Steps
- Coagulation
- Flocculation
- Sedimentation
- Filtration
Coagulation - Coagulation is often the first step in water treatment. During coagulation, chemicals with a positive charge neutralize the negative charge of dirt and other dissolved particles in the water. When this occurs, the particles bind with the chemicals to form slightly larger particles. Common chemicals used in this step include specific types of salts, aluminum, or iron.
Flocculation - Flocculation follows the coagulation step. Flocculation is the gentle mixing of the water to form larger, heavier particles called flocs. Often, water treatment plants will add additional chemicals during this step to help the flocs form.
Sedimentation - Sedimentation is one of the steps water treatment plants use to separate solids from the water. During sedimentation, flocs settle to the bottom of the water because they are heavier than water.
Filtration - Once the flocs have settled to the bottom of the water, the clear water on top is filtered to separate additional solids from the water. During filtration, the clear water passes through filters with different pore sizes and made of various materials, such as sand, gravel, and charcoal. These filters remove dissolved particles and germs, including dust, chemicals, parasites, bacteria, and viruses. Activated carbon filters also eliminate any unpleasant odors.
Water treatment plants can use a process called ultrafiltration in addition to or instead of traditional filtration. During ultrafiltration, the water passes through a filter membrane with very small pores. This filter only allows water and other small molecules, such as salts and tiny charged molecules, to pass through.
Disinfection - After filtration, the water undergoes disinfection to kill any remaining bacteria, viruses, and other pathogens that may be present. Disinfection is crucial for ensuring the safety of drinking water and preventing waterborne diseases.
Common disinfection methods used in water treatment plants include:
- Chlorination: Chlorine-based compounds, such as chlorine gas, sodium hypochlorite, or calcium hypochlorite, are added to the water to kill bacteria and viruses. Chlorine effectively destroys pathogens by disrupting their cellular function.
- Chloramination: Chloramine, a combination of chlorine and ammonia, is another disinfection method used in some water treatment plants. Chloramine is effective in maintaining disinfection residuals throughout the distribution system, providing long-lasting protection against microbial contamination.
- UV Disinfection: Ultraviolet (UV) light is used to disinfect water by damaging the genetic material of microorganisms, preventing them from reproducing. UV disinfection is a chemical-free method that can effectively inactivate a wide range of pathogens.
- Ozonation: Ozone gas is a powerful oxidizing agent that is used to disinfect water by destroying bacteria, viruses, and other microorganisms. Ozone works by reacting with organic and inorganic compounds, effectively eliminating pathogens and reducing the risk of waterborne diseases.
- Chlorine Dioxide Treatment: Chlorine dioxide is a highly effective disinfectant that is used to kill bacteria, viruses, and protozoa in water. It works by disrupting the cellular function of microorganisms, rendering them harmless.
Disinfection is typically the final step in the water treatment process before the water is distributed to consumers. It ensures that the water is safe for drinking and meets regulatory standards for microbial quality.
Domestic Water Purifiers
Household water treatment is treatment of water that happens at home or at a point of use or collection locations within communities (such as schools and community centers). In the absence of a piped water system, this type of treatment can make water safe to use and reduce diarrheal and other waterborne diseases.
When choosing water treatment methods for a household or community setting, several factors must be considered:
- Existing water and sanitation conditions
- Water quality
- Ability to install water, sanitation, and hygiene (WASH) facilities
- Cultural acceptability
- Accessibility
- Availability of technology
- Consistent and long-term use
- Other local conditions
There are various methods to make water safe from harmful germs. The most commonly used methods include:
- Boiling
- Chlorination
- Flocculant - Disinfection
- Solar Disinfection
- Slow Sand Filteration
Boiling: Boiling or heating water is the most widely used and effective method for killing disease-causing germs, including viruses, bacteria, and parasites. The steps for boiling water are as follows:
- Bring clear water to a rolling boil for 1 minute (at elevations above 6,500 feet, boil for 3 minutes).
- After boiling, allow the water to cool before use.
- Store the boiled water in clean, sanitized containers with tight covers.
Chlorination: Chemical disinfection is another common method for making water safe to use. Chlorination involves adding a chlorine-based product, such as sodium hypochlorite, calcium hypochlorite, or household bleach, to water to kill bacteria and viruses. Other chemical disinfectants, such as iodine and chlorine dioxide, can also be effective for disinfecting water. Using or drinking water with small amounts of chlorine, iodine, or chlorine dioxide does not cause harmful health effects and provides protection against waterborne disease outbreaks.
While chlorine products can kill most harmful or disease-causing viruses and bacteria, most disinfectants are not as effective as boiling for killing more resistant germs, such as the parasites Cryptosporidium and Giardia. Chlorine dioxide tablets can kill Cryptosporidium if you follow the manufacturer's instructions correctly.
Flocculant-Disinfection: Flocculation-disinfection is a water treatment process where a product is added to water, causing solids to form larger clusters, or flocs, that can be removed from the water.
PUR sachets are a widely used flocculant-disinfectant powder. The PUR product contains powdered ferric sulfate (a flocculant) and calcium hypochlorite (a disinfectant). To use, add the contents of one PUR sachet to a 10-liter volume of water and stir for 5 minutes. After stirring, solids settle to the bottom of the container, and the water can be poured through a cloth filter into a secondary water container for safe storage and use. Note: For residual protection, there is also chlorine in PUR.
This method is most appropriate when water is turbid (cloudy).
Solar Disinfection: Solar disinfection is a method that uses heat and UV radiation to kill bacteria and parasites in water. To use solar disinfection place contaminated water in a transparent container and expose it to strong sunlight for 6 to 8 hours if sunny, or 2 days if cloudy.
This method is most appropriate when water is clear, and clean transparent containers for treatment are available.
Slow Sand Filtration: Slow sand filtration effectively removes turbidity (cloudiness) and microorganisms through various biological and physical processes in a single treatment step. A slow sand filter consists of vertically arranged layers of components. When constructed, the filter includes a tank, a bed of fine sand, a layer of gravel to support the sand, a system of underdrains to collect the filtered water, and a flow regulator to control the filtration rate. No chemicals are added to aid in this filtration process.
Applied Chemistry
Applied Chemistry HPTU BTech CSE Syllabus
Electrochemistry
Specific, equivalent and molar conductivity of electrolytic solutions, Reference Electrodes-Calomel electrode and Ag-AgCl electrode, Ion-selective electrode-Glass electrode, determination of pH of solution using glass electrode, Construction and working of Batteries-Lead acid storage battery, Ni-Cd storage cell, Lithium batteries, fuel cell and Solar cell.