Wave Function
Waves, in general, are associated with quantities that vary periodically. In the case of matter waves, the quantity that varies periodically is called the wave function.
The wave function, represented by , associated with matter waves has no direct physical significance as an observable quantity. However, the value of the wave function is related to the probability of finding the particle at a given place at a given time.
The square of the absolute magnitude of the wave function of a particle, evaluated at a particular time and place, is proportional to the probability of finding the particle at that place at that instant.
The wave functions are usually complex. The probability density in such a case is given by , i.e., the product of the wave function with its complex conjugate, being the complex conjugate. Since the probability of finding a particle somewhere is finite, we have the total probability over all space equal to unity. That is
where
This equation is called the normalization condition, and a wave function that obeys this equation is said to be normalized. Further, must be single-valued since the probability can have only one value at a particular place and time. Besides being normalizable, a further condition that must obey is that it and its partial derivatives, , and be continuous everywhere.
The important characteristics of the wave function are as follows:
- must be finite, continuous and single-valued everywhere.
- , and must be finite, continuous and single valued.
- must be normalisable.
Postulates of Quantum Mechanics
-
State Postulate
A physical system is completely described by its wave function (state function) . The wave function is a complex-valued function of the coordinates of the particles and time.
- contains all the information about the system.
- The probability density function is given by .
-
Observable Postulate
Every observable in quantum mechanics is represented by a corresponding operator. These operators are Hermitian to ensure real eigenvalues, which correspond to measurable quantities.
- For example, the position observable is represented by the operator , and the momentum observable by .
-
Measurement Postulate
The only possible result of a measurement of an observable is one of the eigenvalues of the corresponding operator.
- When a measurement is made, the system collapses to an eigenstate of the observable, and the measurement outcome is the eigenvalue associated with that eigenstate.
-
Expectation Value Postulate
The expectation value (average measurement) of an observable in a state is given by:
-
Time Evolution Postulate
The time evolution of the wave function is governed by the Schrödinger equation:
where is the Hamiltonian operator, representing the total energy of the system.
Schrödinger Equation
It is not a single video but a playlist, and it contains three videos. When one video finishes, the next will play automatically.
The Schrödinger Equation is a mathematical expression that describes the change of a physical quantity over time in scenarios where quantum effects, such as wave-particle duality, are significant. There are two forms of the Schrödinger Equation: the time-dependent Schrödinger Equation and the time-independent Schrödinger Equation.
Derivation of Schrodinger Equation for Time Independent
Consider a system of stationary waves associated with a moving particle. The waves are said to be stationary with respect to the particle. If the position coordinates of the particle are and is the periodic displacement for the matter waves at any instant of time , then we can represent the motion of the wave by a differential equation as follows:
where is the velocity of wave associated with the particle. The solution of Eq. (i) gives as a periodic displacement in terms of time, i.e.,
where is the amplitude of the particle wave at the point (x, y, z) which is independent of time (t). It is a function of (x, y, z), i.e., the position and not of time . Here,
Eq. (ii) may be expressed as
Differentiating Eq. (iv) twice with respect to , we get
or
Substituting the value of from this equation in Eq. (i), we get
where [as ]
So that
Also,
where is known as Laplacian operator. Using Eqs. (vi), (vii) and (viii), we have
Also from the deBroglie wave concept,
Using this relation in Eq. (ix) gives
Here it can be noted that the velocity of particle has been introduced in the wave equation. If and are respectively the total energy and potential energy of the particle then its kinetic energy is given by
The use of Eq. (xii) in Eq. (xi) gives rise to
or
This is the time independent Schrödinger equation, where the quantity is known as wave function. For a freely moving or free particle . Therefore, Eq. (xii) becomes
Derivation of Schrodinger Equation for Time Dependent
To obtain the time-dependent Schrödinger equation, we start from the time-independent Schrödinger equation and reintroduce the time dependence. We differentiate Eq. (iv) with respect to and obtain:
or
Substituting the value of from Eq. (xiv) in Eq. (xii), we have
or
or
This equation is known as Schrödinger’s time dependent wave equation. The operator is called Hamiltonian operator and is represented by .
Therefore, the Schrödinger equation can be written in operator form as:
And in terms of the Hamiltonian operator :
Here,
Application viz. Particle in One Dimensional Box
Schrödinger's equation is extremely useful for investigating various quantum mechanical problems. By applying this equation with appropriate boundary conditions, we can obtain the expression for the wave function and then calculate the probability of finding the particle using the wave function. In this subsection, we discuss the quantum mechanical problem of a particle in a box, also known as the infinite potential well.
Particle in a Box (Infinite Potential Well)
The simplest quantum mechanical problem is that of a particle trapped in a box with infinitely hard walls. Infinitely hard walls mean the particle does not lose energy upon collision with the walls, maintaining constant total energy. A physical example of this problem could be a molecule confined within a box.
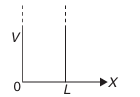
Consider a particle restricted to move along the x-axis between and , by ideally reflecting, infinitely high walls of the infinite potential well, as shown in Fig. Suppose that the potential energy of the particle is zero inside the box, but rises to infinity outside, that is,
for (inside the box)
for and (outside the box)
In this case, the particle is said to be moving in an infinitely deep potential well. To evaluate the wave function in the potential well (where ), Schrödinger's equation is written as:
By setting , the equation becomes:
The general solution of this differential equation is:
where and are constants.
Applying the boundary condition at , we obtain:
With at , we have:
This equation is satisfied when:
or
or
or
or in general we can write Eq. (v) as
Therefore, in an infinite potential well, the particle cannot have arbitrary energy but only certain discrete energy values corresponding to . These are called the eigenvalues of the particle in the well and constitute the energy levels of the system. The integer corresponding to the energy level is called its quantum number as shown in fig below.
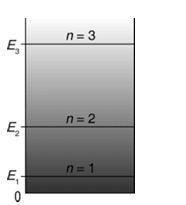
The wave function (or eigen function) is given by Eq. (iii) along with the use of expression for
or